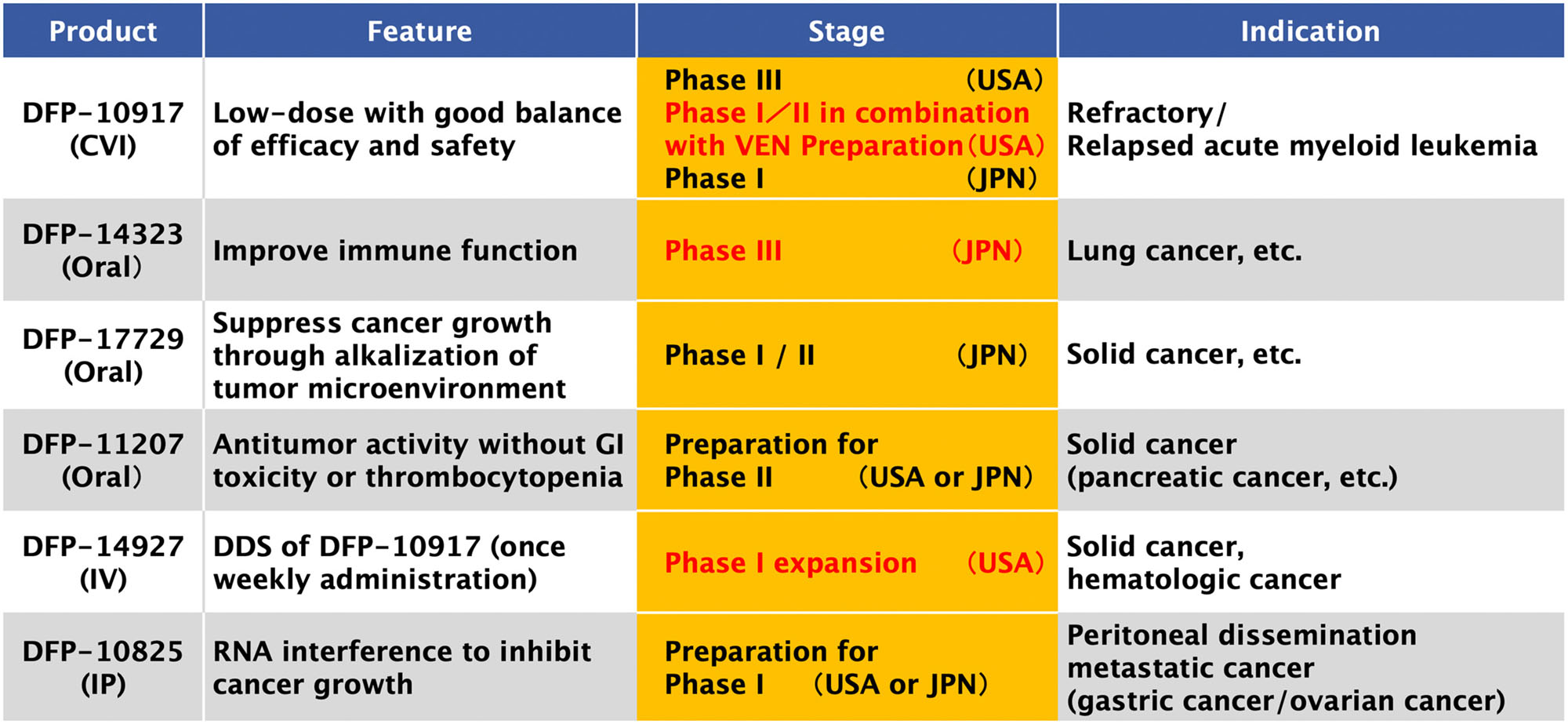
1) DFP-10917
- Indication
- Relapsed/refractory acute myeloid leukemia
- Feature
- Nucleoside analog delivered as a low-dose continuous infusion

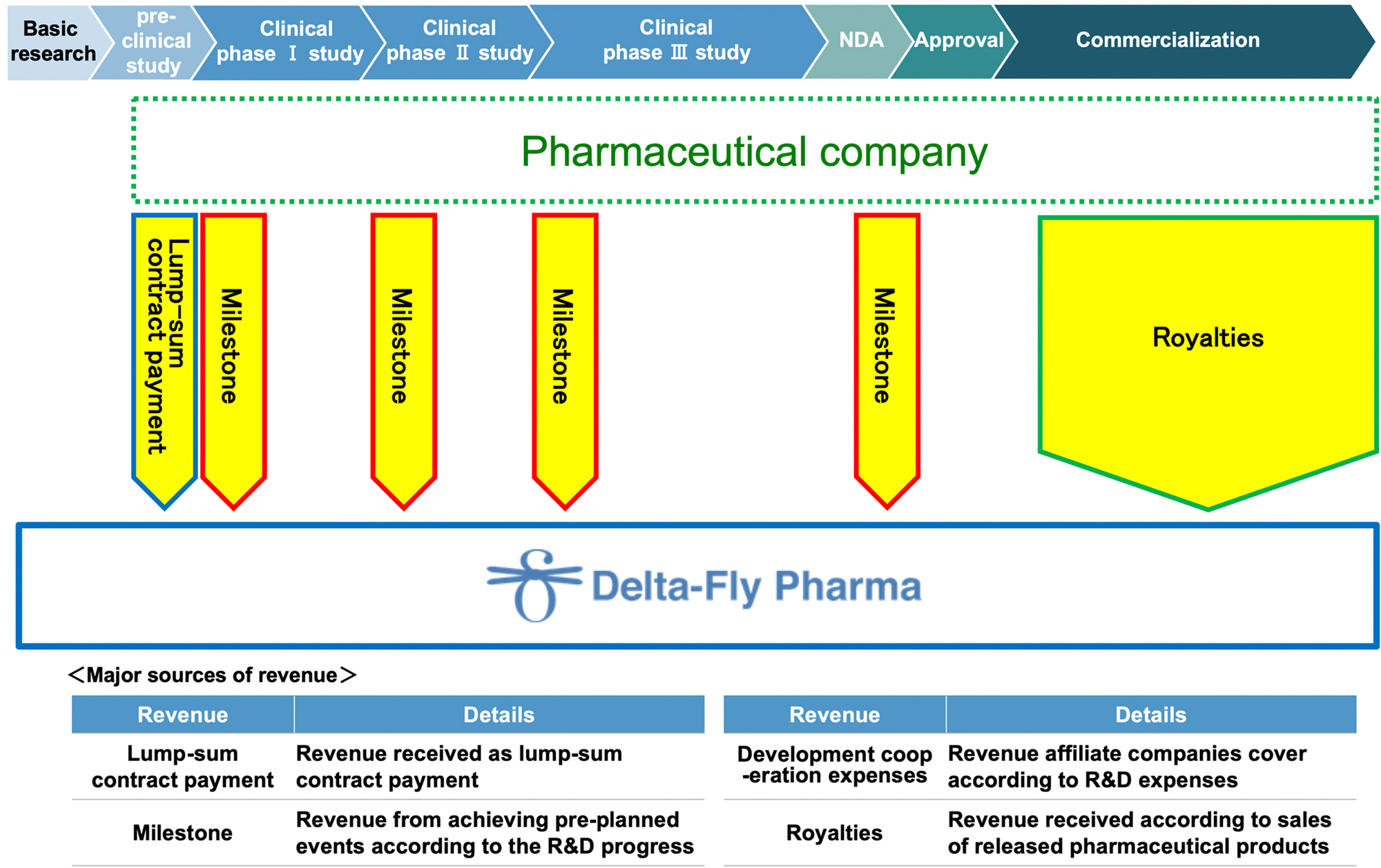

| Product | Partner | Country | Contract Rights | Contract Date | Contract Type | Contract Term |
|---|---|---|---|---|---|---|
| DFP-10917 | Nippon Shinyaku Co., Ltd. | JPN | Manufacturing and marketing | March 24, 2017 | Exclusive | Upon Japan patent expiry or 15 years from first sales, whichever is later. |
| DFP-17729 | Nippon Chemiphar Co., Ltd. | JPN | Manufacturing and marketing | March 26, 2020 | Exclusive | Upon termination of sales by Nippon Chemiphar or sub-licensee. |
| DFP-14323 | Nippon Chemiphar Co., Ltd. | JPN | Manufacturing and marketing | March 8, 2022 | Exclusive | Upon termination of sales by Nippon Chemiphar or sub-licensee. |